Batteries are a crucial part of modern energy storage technologies, powering anything from electric cars to portable electronics. As demand for efficient energy storage grows, advancements in battery chemistry and design are essential for better performance and supporting the shift to sustainable energy.
Introduction:
In this study, we focus on lithium-ion polymer (Li-Po) batteries, a type of rechargeable battery that utilizes a polymer electrolyte. Li-Po batteries are valued for their low density, high specific capacity, and strong electrochemical performance, making them ideal for high-efficiency applications.
We employed Galvanostatic Charge/Discharge (GCD) testing to assess battery functionality. This method, which involves applying constant current during charging and discharging cycles, provides insights into the battery’s performance by evaluating capacitance, Charging and Discharging Capacity, energy efficiency, and power efficiency for multiple cycles.
What you will learn in this Application Note?
- What are Li-ion batteries?
- What are Li-ion batteries, and how do they work?
- How to perform safety checks during battery testing?
- How to determine the nominal voltage of a Li-ion battery?
- What is the significance of the Time Limit in GCD (Galvanostatic Charge/Discharge) techniques?
- How to analyze and interpret charging and discharging curves?
Apparatus Required:
In our experiments, we used MedPstat 2.0 to test a 100mAh Li-Po battery with a nominal voltage of 3.2V. All measurements and calculations were performed using specific software provided by the company, ensuring precise control and data analysis.

Experimental:
Before starting the experiment, it is essential to connect the battery terminals to the Medpstat 2.0 instrument. To do this, first convert the 3-electrode system into a 2-electrode configuration by short-circuiting the reference electrode with the counter electrode (refer to Fig. 1 for a visual guide). When connecting the battery, ensure that the red wire is connected to the Working Electrode, while the black wire is connected to both the Counter and Reference Electrodes (Fig 2).

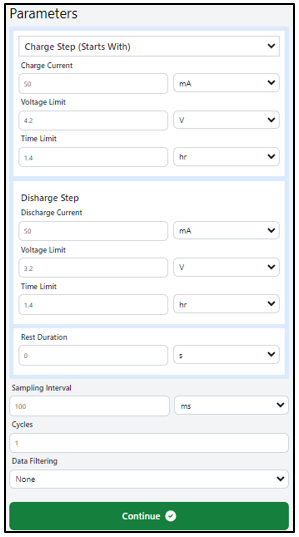
Once the instrument is successfully connected to the battery, open the MedEPlot software 2.0 and select the Galvanostatic Charge Discharge Technique. Depending on your experimental goal, you can choose either the charging or discharging option first. Here, we begin with the charging phase, followed by discharging, with a rest period of 5 seconds between the charging and discharging steps. For more details on setting up the parameters, refer to Fig. 3 in the “Experimental Parameters” section.
Experimental Parameters:
Result and Discussion:
Run an Open Circuit Potential (OCP) test to verify the correct connection of the terminals. So, Perform the OCP test for 10 seconds, ensuring that the measured voltage remains positive, which confirms that the experimental setup is correctly aligned.
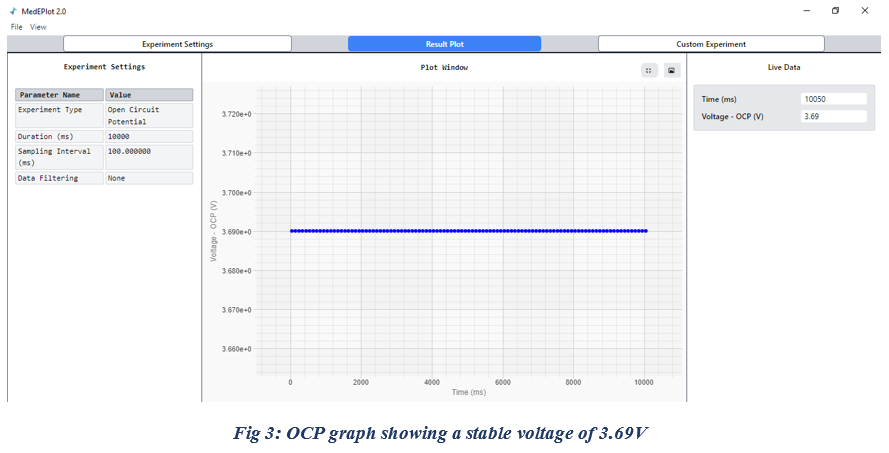
Subsequently, verify the battery voltage by comparing the OCP value with the nominal battery voltage, which is typically around 3.7 V. In this case, the OCP reading was approximately 3.69 V (Fig 3), indicating that the battery is in good condition. However, significant deviations from the nominal value may suggest battery degradation or failure.
Given that our Li-Po battery has a capacity of 100mAh, it is important to ensure the current remains below this value to prevent potential hazards, such as battery failure or explosion. In this experiment, we will apply a charging and discharging current of 50mA. The charging voltage limit (cutoff voltage) is set at 4.2V, while the discharging limit is set at 3.2V. Adhering to the discharging limit is critical, as over-discharging can damage the battery or pose safety risks. To maintain control, we will set a time limit of 1.4 hours for both the charging and discharging cycles.
To begin, we will perform the charging process. Select the charging option, and then set the parameters as specified above, or enter your desired current and voltage. Once the parameters are configured, click the “Continue” button to initiate the experiment.
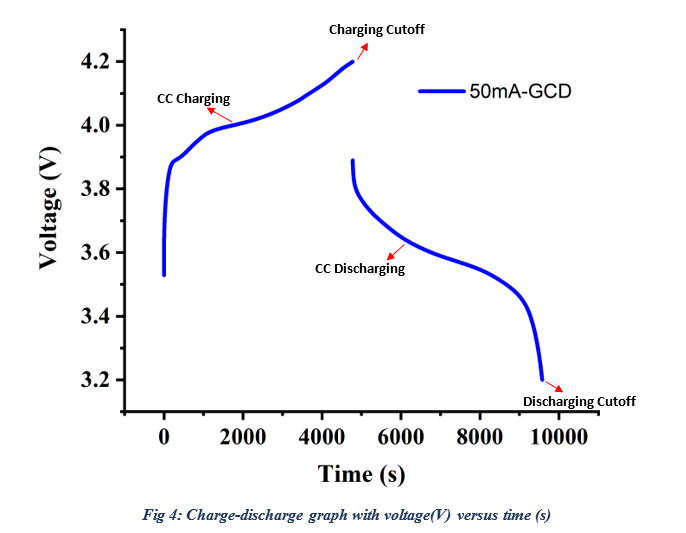
During the charging process (Fig: 4), a graph displaying potential (voltage) versus time will be generated, enabling real-time monitoring of the battery’s charging behavior. On the right side, live data will provide insights into the charge-discharge process. The step count will indicate the process phase: 1 for charging, 2 for rest period, 3 for discharging, and 4 for rest period. This sequence repeats for each cycle. If discharging occurs first, the steps will follow the order of discharge-rest-charge-rest.
Experimental Summary Table:
|
Experimental Name |
Cycle Number | Capacity Charge(mAh) | Capacity Discharge(mAh) | Coulombic Efficiency(%) | Energy Efficiency(%) |
| Galvanostatic Charge Discharge | 1 | 66.39 | 66.33 | 100 |
76.46 |
Conclusion:
In conclusion, this application note highlights key practical aspects of Li-ion battery technology particularly in testing the batteries’ nominal voltage using Open Circuit Potential (OCP) and analyzing the charge-discharge process. Through the evaluation of charging and discharging capacities, as well as coulombic and energy efficiencies, the study offers valuable insights into the real-world performance of Li-ion batteries. These findings contribute to a deeper understanding of how to optimize battery usage for reliable energy storage.
Thanks for Choosing MedPstat as your research companion.