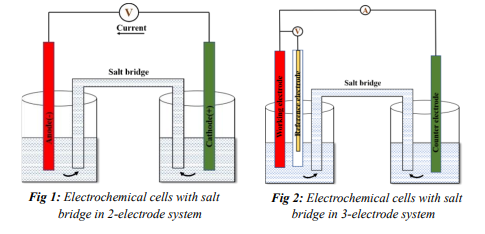
When the electrodes are in a single container, an electrochemical cell can function without a salt bridge, because electron flow happens naturally within the system. However, if the electrode is situated in two different containers, as shown in Fig 1 and Fig 2, the salt bridge aids in transferring charges from one electrolyte to another. Inert electrolytes such as KCl or NaOH must be used to fill the salt bridge. These electrolytes have similar migration rates and conductivities, and they do not participate in any chemical reactions in electrolytic cells.