What is a Reference electrode?
A reference electrode serves as a fixed benchmark for measuring the potential of other electrodes in electrochemical systems. Essentially, it’s an electrode with a precisely known and stable half-cell potential. Importantly, this potential remains consistent regardless of the solution’s composition. Think of it as a reference point, a bit like a ruler for voltage in chemistry.
In electrochemical setups, this reference electrode can play two roles. Depending on the specific electrodes it’s paired with, it can act as either the anode (where oxidation occurs) or the cathode (where reduction occurs). This flexibility makes it a valuable tool for scientists and engineers to gauge and control the electrochemical reactions happening in various experiments and applications.
Types of reference electrodes:
- Silver/silver chloride electrode
- Saturated calomel electrode
- Mercury/mercuric oxide electrode
- Copper-copper (II) sulfate electrode
- Mercury-mercurous sulfate electrode
- Ag/Ag+ electrode (non-aqueous)
Applications of reference electrodes
- Corrosion studies
- pH measurements
- batteries
- fuels cells
- electroplating
- electrodeposition
- electrochemical sensors and electroanalytical studies like potentiometry, voltammetry and amperometry.
These reference electrodes differ in their composition and the way their potentials are determined. The choice of reference electrode depends on the specific requirements of the electrochemical experiment and the electrolyte conditions, such as pH and ionic composition.
1.Ag/AgCl Electrode:
The Ag/AgCl reference electrode is a key tool in electrochemical research. It’s made of a silver wire coated with silver chloride, immersed in a potassium chloride solution. This electrode is super stable, doesn’t drift much, and stays reliable for a long time.
In experiments, it’s part of a three-electrode system with a working electrode (where reactions happen) and a counter electrode (to complete the circuit). The Ag/AgCl electrode is like the “zero point” for voltage at 25°C.
The electrode can be represented as:
Ag, AgCl |Cl-
The half-cell reaction of Ag/AgCl electrode:
AgCl(s)+e– ⇋ Ag(s) +cl–
For this half-cell reaction Gibbs free energy is negative, so the reaction is spontaneous reaction.
Reference electrode potentials:
| ELECTRODE | POTENTIAL(V) |
| Ag/AgCl/saturated KCl | +0.197 |
| Ag/AgCl/3.5 mol/kg KCl | +0.205 |
| Ag/AgCl/3.0 mol/kg KCl | +0.210 |
| Ag/AgCl/1.0 mol/kg KCl | +0.235 |
| Ag/AgCl/0.6 mol/kg KCl | +0.250 |
| Ag/AgCl (sea water) | +0.266 |
Reference: “NACE International CP Specialist Course Manual”
Applications
- Biological and Physiological Studies: Ag/AgCl electrodes are commonly used in biomedical and physiological research for measuring the electrical activity of cells and tissues (e.g., electrocardiography).
- Chloride-Containing Solutions: They are ideal for solutions with chloride ions, such as seawater or saline solutions.
Advantages:
- Compatibility with chloride-containing solutions.
- Stable potential in a wide range of applications.
Limitations:
- Can be affected by contamination or precipitation of silver chloride on the electrode surface.
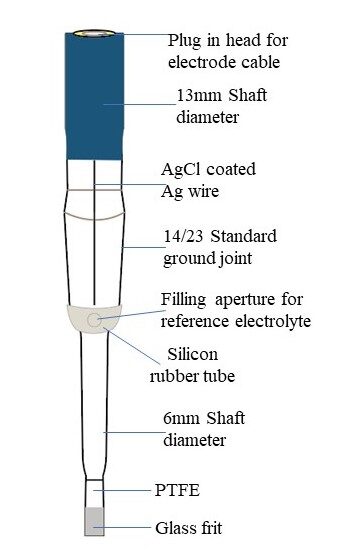
Fig 1: Ag/AgCl reference electrode
2. Saturated Calomel Electrode (SCE):
- Composition: Consists of a mercury (Hg) electrode immersed in a saturated solution of potassium chloride (KCl) with mercury(I) chloride (Hg2Cl2) as a solid-state electrolyte.
- Potential: The standard potential of SCE is +0.242 V vs. the Standard Hydrogen Electrode (SHE).
The electrode can be represented as:
Hg, Hg2Cl2 |Cl-
The half-cell reaction of Ag/AgCl electrode:
Hg2Cl2 (S)+2e– ⇋ 2Hg(l) +2Cl–
Application: SCE is widely used in many electrochemical experiments and measurements due to its stable and well-defined potential. It is commonly used in laboratories for various electrochemical applications.
Advantages:
- Reliable and stable potential.
- Suitable for a wide range of electrochemical applications.
Limitations:
- Contains toxic mercury, which can be hazardous.
- Incompatible with some solutions containing strong reducing agents.

Fig 2: SCE reference electrode
3. Hg/HgO Electrode
- Composition: Comprises a mercury (Hg) electrode in contact with mercury (II) oxide (HgO) in a potassium hydroxide (KOH) electrolyte solution.
- Potential:
The potential of the Hg/HgO electrode depends on the pH of the KOH electrolyte and can be calculated using the Nernst equation.
The electrode can be represented as:
HgO, H2O |OH–
The half-cell reaction of Ag/AgCl electrode:
HgO + H2O + 2e– ⇋ Hg + 2OH–
Applications:
- Alkaline Environments: Hg/HgO electrodes are preferred in alkaline solutions and are often used for pH measurements in alkaline media.
- Corrosion Studies: They are employed in studying corrosion processes, especially in high-pH environments.
Advantages:
- Suitable for alkaline solutions.
- Can be used in studies involving high pH levels.
Limitations:
- pH-dependent potential requires careful calibration.
- Mercury-based electrodes have environmental concerns.
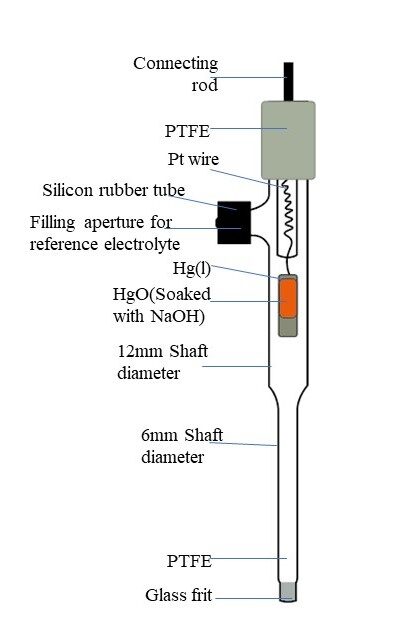
Fig 3: Mercury/mercuric oxide reference electrode
4. Cu/ CuSO4 Electrode:
The Cu/CuSO4 reference electrode is commonly employed in electrochemical studies that involve copper-based systems or require a stable reference potential based on copper. It consists of a copper (Cu) electrode immersed in a saturated solution of copper sulfate (CuSO4). This electrode is particularly useful when working with copper electrodeposition, corrosion, or any electrochemical process where a copper reference potential is desired.
Standard Potential: The standard potential of the Cu/CuSO4 reference electrode is approximately +0.337 V vs. the Standard Hydrogen Electrode (SHE) at 25°C.
The electrode can be represented as:
CuSO4, Cu+|SO42-
The half-cell reaction of Cu/CuSO4 electrode:
CuSO4+2e– ⇋ Cu+ + SO42-
Application: The Cu/CuSO4 reference electrode is frequently used in studies involving copper-based electrochemical systems, such as copper electrodeposition, corrosion of copper materials, and investigations into copper redox reactions. It is also suitable for experiments where a stable reference potential based on copper is needed.
5.Hg/Hg2SO4 Electrode:
The Hg/Hg2SO4 reference electrode is utilized in electrochemical experiments that require a stable reference potential based on mercury. It consists of a mercury (Hg) electrode in contact with a saturated solution of mercury(I) sulfate (Hg2SO4). This reference electrode is chosen when working with mercury electrochemistry, amalgamation processes, or other situations where a mercury-based reference potential is needed.
Standard Potential: The standard potential of the Hg/Hg2SO4 reference electrode is approximately +0.7 V vs. the standard Hydrogen Electrode (SHE) at 25°C.
The electrode can be represented as:
Hg2SO4, Hg+|SO42-
The half-cell reaction of Cu/CuSO4 electrode:
Hg2SO4 +2e– ⇋ 2Hg + SO42-
Application:
The Hg/Hg2SO4 reference electrode is chosen when a stable reference potential based on mercury is required. It is employed in experiments involving mercury electrochemistry, such as investigations into amalgamation processes and other mercury-related reactions
6.Ag/Ag+ Electrode:
- Composition: Comprises a silver (Ag) electrode in contact with a solution containing silver ions (Ag+).
- Potential: The potential of the Ag/Ag+ electrode depends on the concentration of silver ions and can be calculated using the Nernst equation.
The electrode can be represented as:
AgNO3|Ag|NO3
The half-cell reaction of Ag/Ag+ electrode:
AgNO3 + e– ⇋ Ag(s) + NO3
Application: Ag/Ag+ electrodes are used when the specific concentration of silver ions in the electrolyte needs to be controlled, such as in ion-selective electrode measurements and certain analytical techniques.
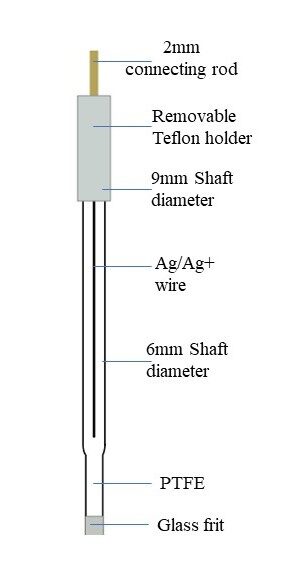
Fig 4: Ag/Ag+ reference electrode
Factors which affect the function of reference electrode: –
- The filling solution -always needs to be checked to be clear without any crystal or precipitation
- Bubbles -make sure that there is not any bubble inside the electrode
- Porous frit-sometimes it is contaminated, clogged or broken
Standard electrolyte solution:
- Make sure it won’t mess with or pollute your sample.
- The filling solution should have the most important ions needed at the junction.
- Try to have the ions in the filling solution move at the same speed, both the positive and negative ones.
How do I use my electrode?
Reference electrode is used to perform electrochemical measurements through various techniques such as cyclic voltammetry, chronoamperometry, impedance spectroscopy etc.
In the utilization of a reference electrode, there are two different ways it can be used: one with a salt bridge and another without a salt bridge.
 Electrochemical cell setup with stand Gas tight conical cell with stand
Electrochemical cell setup with stand Gas tight conical cell with stand
Designs of reference electrodes:
Three different designs of reference electrodes are available in our MTX products:
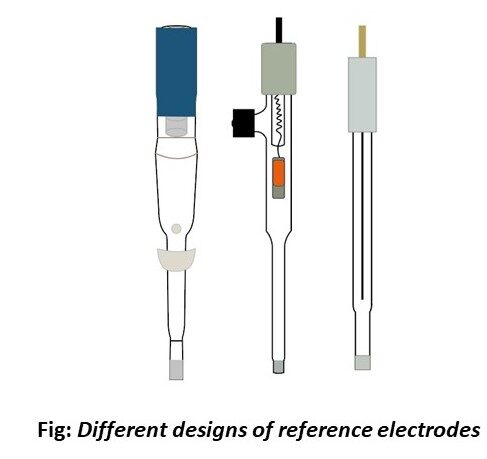
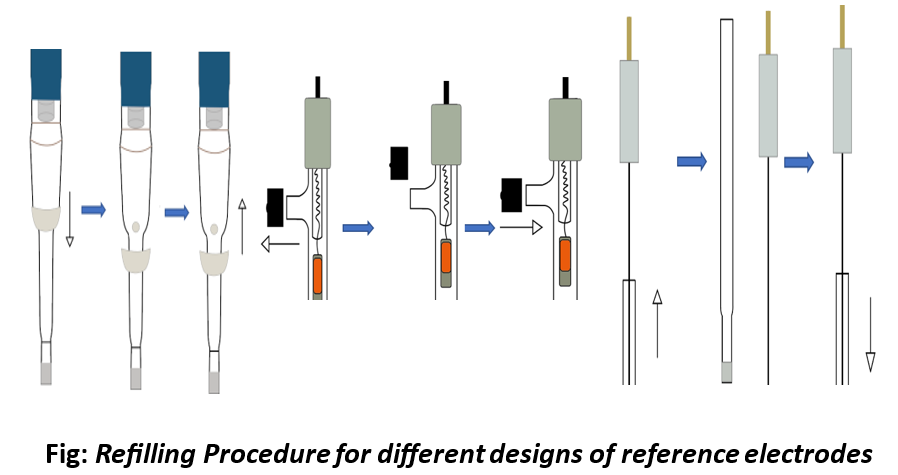
How do I refill my reference electrodes for different designs?
- Hold the glass tube in one hand.
- With your other hand, gently push the silicon rubber tube (refer figures) to remove it from the filling hole
- Use a syringe to refill the electrolyte compartment.
- Fill it completely; ensure there are no air bubbles.
- Excessive pressure can harm the porous glass filter (diaphragms).
- After completing the filling, close the filling port by pushing silicone rubber.
Q. How can the reference electrodes functionality be tested?
A, A basic voltmeter can be used to measure the potential, which can then be compared to a standard reference electrode such as a Saturated Calomel Electrode (SCE) or Silver/Silver Chloride (Ag/AgCl) and a cell containing 3M KCl.
Q. How should reference electrodes be appropriately stored to maintain their performance and longevity?
A. To maintain the performance and longevity of reference electrodes, follow these key practice s for appropriate storage:
- Keep it Wet: Store the reference electrode in the recommended filling solution to keep the reference element hydrated.
- Seal and Cap: Ensure any removable caps or seals are tightly closed to prevent evaporation and contamination.
- Proper Container: Use a clean and dry container free from contaminants.
- Avoid Air Exposure: Minimize air exposure to prevent depletion of the filling solution.
- Regular Maintenance: Periodically check the filling solution level, cleanliness, and replace it when necessary.