Electrochemical DNA Sensors
Electrochemical detection of DNA is of high interest to the scientific community and the electronics, semiconductor, and medical devices industries. Electrochemistry-based sensors are precise, highly selective, and don’t cost much to produce. This is made possible by the successful interactions between the target molecule, the recognition layer, and a solid electrode surface. There have been many methods developed to achieve this. Let’s look at some of them below!
Let’s explore the following areas
- What does Electrochemical Sensing have to Offer in DNA Research?
- The Science Behind Biosensors
- Electrochemical biosensors for detection of DNA damage
What does Electrochemical Sensing have to Offer in DNA Research?

You may have heard of ‘gene chips’ or ‘DNA chips’. These dense arrays of oligonucleotides are fixated on a solid surface and have been useful in single-nucleotide polymorphism (SNP) discovery. Expensive instrumentation to analyze the readout of these chips and complex algorithms to interpret the data limits their use in genetic research.
DNA-based electrochemical sensing combines layers of nucleic acid with electrochemical transducers which is what we call a ‘biosensor’. As a result, this solves our problem of an option that is inexpensive, simple to use, and accurate in results for clinical purposes.
Why do we need Electrochemistry in Genetic Research?
DNA is a prime target for drugs. Drugs can alter the structure and sequence, disrupting DNA replication and contributing to cancer. Therefore, this drives the need for affordable, adaptable tools for detecting DNA damage, thus advancing DNA biosensor technology.
DNA biosensors are integrated receptor-transducer devices where DNA is the recognition element to detect specific binding events, typically through electrical, thermal, or optical signal transduction.
The Science Behind Biosensors

Electrochemical transducers combine the advantages of DNA probes with direct, label-free detection, making them useful in various applications, such as monitoring environmental pollutants, studying DNA-drug interactions, detecting DNA damage in clinical settings, and identifying specific DNA sequences in nucleic acids from humans, viruses, and bacteria. Among these, carbon electrodes—like glassy carbon, carbon fiber, graphite, and carbon black—are notable for their wide electrochemical potential window, which enables sensitive detection of DNA damage by observing oxidation peaks in DNA bases.
For a biosensor, a recognition step is necessary for it to detect the target analytes. The sensor’s entire role is to ensure the probe-target complex formation takes place and thus the binding step will set in motion a signal for analytical readout. The biosensor must have a molecular recognition layer and a signal transducer that can be put together with a proper readout device.
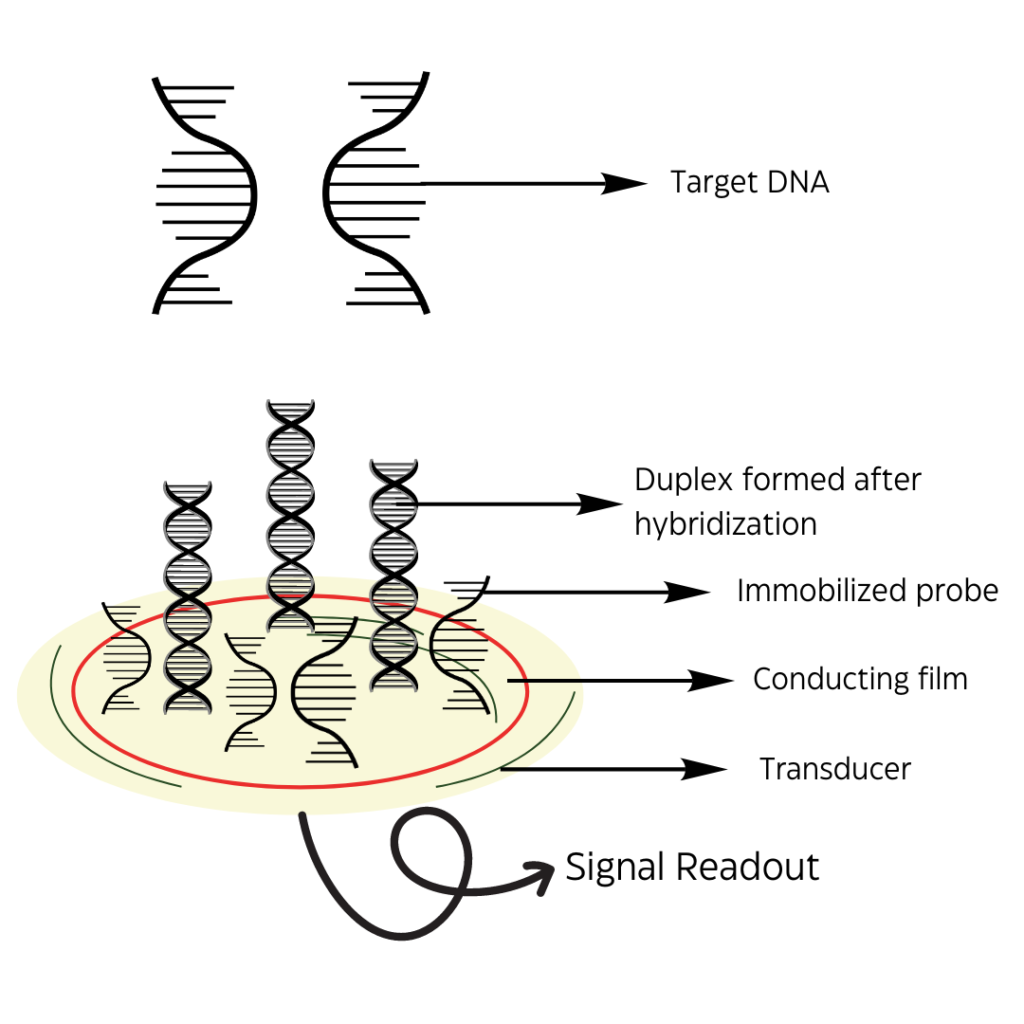
Figure 1: Representation of a general electrochemical DNA sensor attached to a signal readout device.
DNA serves as a robust participant for biosensing as the interactions between the base pairs are complementary and specific. Electrochemical oxidation of DNA at a Glassy Carbon Electrode (GCE) using differential pulse voltammetry reveals two current peaks corresponding to the oxidation of guanine and adenine residues. The electrochemical behavior of double-stranded DNA (dsDNA) and single-stranded DNA (ssDNA) shows that electron transfer is more challenging for dsDNA due to its rigid structure, which makes it harder for electrons to move from within the double helix to the electrode surface. In contrast, the flexible ssDNA, with its bases closer to the electrode, can more easily navigate the rough surface of the electrode and fit into its grooves.
When natural or synthetic DNA interacts with electrode surfaces, it adsorbs and undergoes conformational changes. Proper DNA immobilization and stabilization on the electrode are key to creating effective DNA-electrochemical biosensors, impacting the DNA probe’s characteristics, sensor response, and overall performance.
A usual setup would look something like this: a single-strand probe immobilized on the recognition layer where the base pairings will employ the target DNA sequence to the surface. Once the recognition step has occurred, it is left up to the signal transduction step on how well it will be captured, either, optically, mechanically, or electrochemically.
The electrochemical method is preferred for DNA testing and diagnosis as the electrochemical reaction that occurs gives a direct electronic signal to the reader hence eliminating the need for any expensive equipment.
1. Direct Electrochemistry of DNA
The reduction and oxidation of DNA at a mercury electrode; the amount of DNA reduced or oxidized would reflect the amount of arrested DNA. The purine bases of DNA can be oxidized by using carbon, gold, Indium Tin Oxide (ITO), and polymer-coated electrodes.
This methodology is quite sensitive and is limited by background currents at the high potentials required for direct DNA oxidation.
2. Indirect electrochemistry of DNA
DNA can be oxidized indirectly through the use of electrochemical mediators.
- One approach uses polypyridyl complexes of Ru(II) and Os(II) to mediate the electrochemical oxidation of guanine. This technique has been coupled to a reverse transcription–PCR assay to monitor the overexpression of genes in tumor samples.
- A coding method wherein chemically modified bases are incorporated into PCR products and the resulting DNA is detected at an ITO electrode by catalytic oxidation of the modified base. Though sensitive, it is not well suited to clinical diagnostics usages yet.
3. DNA-specific redox indicator detection platforms
Target DNA sequences are labeled with redox-active reporter molecules. Appearance of the electrochemical response therefore signals the hybridization event.
Another way involves a three-part ‘sandwich’ assay, in which the redox label has been attached to a synthetic sequence specifically designed to bind an overhang portion of the probe-target complex. Many applications of DNA sensing (for example, real-time pathogen detection) involve tiny amounts of target analytes, with proportionally few hybridization events.
Electrochemical biosensors for detection of DNA damage

Hundreds of compounds interact with DNA and toxic chemicals that interact eventually damage DNA and thus may cause cancer. Understanding the factors of these interactions is important. Once we know this, we can design sequence-specific DNA-binding molecules for chemotherapy and elucidate the mechanisms of action for anticancer drugs.
Metal ions are found in body fluids and can form complexes with DNA and nucleotides. Some of these metal ions are known to cause cancer because they damage DNA and interfere with DNA replication. Metals can react with harmful molecules like superoxide and hydrogen peroxide to produce very reactive substances, such as hydroxyl radicals. These reactive substances can then damage DNA, leading to metal-related oxidative damage.
Platinum-based drugs, such as carboplatin, are known for their ability to inhibit DNA synthesis by forming covalent bonds with DNA. This binding is irreversible and can lead to side effects. A DNA biosensor has been effectively used to measure carboplatin levels in the serum of ovarian cancer patients undergoing treatment. The interaction mechanism of carboplatin with DNA shows that, at low concentrations, it tends to bind more with adenine than with guanine. Understanding this preference helps clarify how platinum-based cancer drugs interact with DNA.
In an electrochemical study examining the interaction between DNA, Cu(II), and quercetin (a flavonoid that acts as a pro-oxidant and exhibits mutagenic properties), researchers used a bare Glassy Carbon Electrode (GCE) and tested solutions containing double-stranded DNA (dsDNA) with either quercetin alone or a quercetin-Cu(II) complex. They found that quercetin alone had only a weak interaction with DNA. However, when Cu(II) ions were added to the DNA-quercetin solution, significant degradation of the DNA helix was observed. Spectrophotometric analyses confirmed that over time, the quercetin-Cu(II) complex caused damage to dsDNA, likely by intercalating with it and disrupting hydrogen bonds.

DNA biosensors are an important tool for the investigation of the electrochemical and biological mechanism of drugs active against cancer.
Thiophene-S-oxides, a recently isolated group of compounds with biological activity against various cancer cells, have been studied for their effects on DNA. Using a glassy carbon electrode modified with a thiophene-S-oxide/dsDNA mixture, in situ electrochemical detection revealed that reduced thiophene-S-oxide interacts with and damages dsDNA, potentially causing strand breaks. Additionally, the thiophene-S-oxide adduct with dsDNA can still be reduced. This study confirmed that a compound/dsDNA film-modified glassy carbon electrode can be used to explore interactions between dsDNA and water-insoluble molecules.
Conclusions
The development of electrochemical DNA biosensors has significantly advanced the sensitive and selective detection of specific interactions between DNA and other compounds that may or may not be toxic. Research in electrochemistry for DNA biosensing devices holds immense promise due to its potential for highly sensitive and selective detection of biomolecular interactions. Electrochemical methods offer rapid, cost-effective, and real-time analysis, which is crucial for advancements in fields such as genetics, environmental monitoring, and medical diagnostics. The ability to detect and quantify specific DNA interactions with high precision can lead to breakthroughs in understanding genetic mutations, identifying biomarkers for diseases, and assessing environmental pollutants.
By leveraging electrochemical techniques, researchers can develop biosensors that not only provide accurate data but also have the potential for miniaturization and integration into portable devices, making them accessible for on-site testing and point-of-care applications.


