Introduction
The analysis of a crime scene using scientific methods has assisted judicial systems around the globe. Forensic evidence can become so significant that it’s best to be cautious when handling evidence. Electrochemistry comes into the scene as instead of removing evidence from the scene of a crime, objective analytical measurements could be done on the spot! The need for on-site forensic techniques is now more than ever.
We will explore the following areas
- An introduction to electrochemical methods
- Voltammetric methods
- Electrochemistry in forensic science with a few examples
Since there is no presence of an all-rounding analytical technique, method, or procedure, it is usually necessary to use a variety of procedures to identify any trace compounds that may be present at a crime scene. Electrochemical techniques provide a comprehensive strategy for necessary selectivity and can measure any chemical species found at a crime scene.
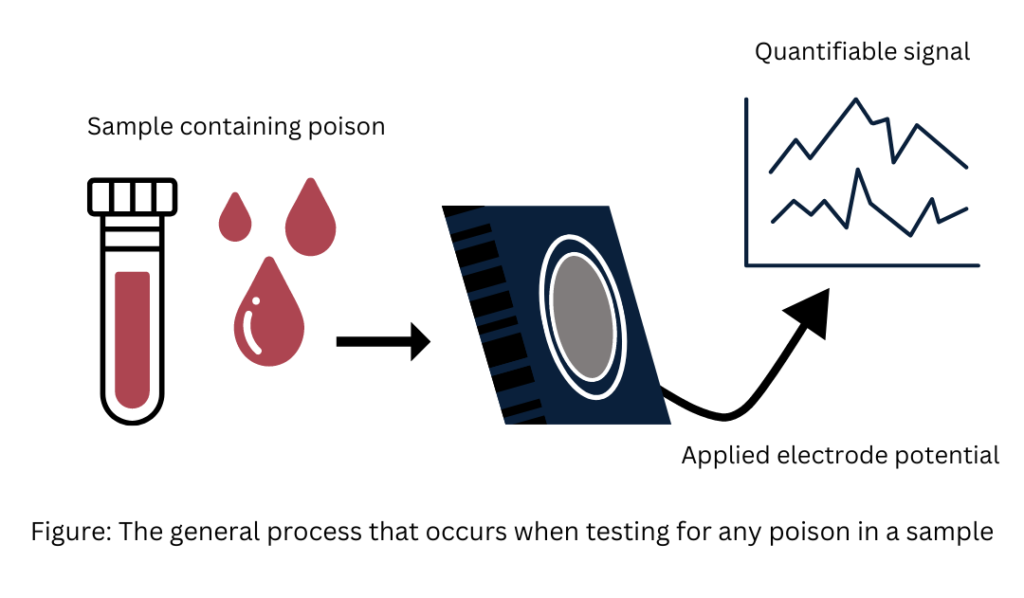
Forensic electrochemistry is the study of the currents (amperes) generated in the oxidation and reduction reactions of chemical species/analytes detected at, around, or about a crime scene.
Researchers employ electroanalytical chemistry, which is characterized by a fixed electrode potential, to track the quantities of chemical species including uric acid, ascorbic acid, and dopamine in a variety of media since the passage of current is directly correlated with the target species’ concentration. Thus, the foundation of forensic electrochemistry is laid by electroanalytical chemistry, which concentrates on the identification of chemical species connected to criminal incidents.
Electrochemical Methods

First, let’s understand some key points in forensic electrochemistry.
The Potentiostatic Method involves the use of a potentiostat, an instrument that forces a change in voltage away from the open-circuit potential to initiate oxidation or reduction reactions in an electrolytic cell. A three-electrode arrangement with a working electrode, a counter electrode, and a reference electrode is typically used to connect the potentiostat to a sample solution.
However, in the absence of a reference electrode, the potential of the reactions would be random. For this reason, a reference electrode offers a well-defined redox pair with a fixed potential, such as the saturated calomel electrode, or SCE. The oxidation and reduction potential is therefore contrasted with the potential of the reactions taking place at the electrode of reference. These potentials and currents, when combined and examined analytically, are what makes the study of electrochemistry so intriguing from analytical chemical interactions.
Let’s look into voltammetric techniques, a popular subset of dynamic electrochemical measurements.
Voltammetric Methods
This method incorporates a potentiostat and a three-electrode electrolytic cell. A potential ramp with a fixed potential step (known as the scan rate) is applied to an electrolytic cell.
The surface interacts with the sample environment via the forces of the electric field. This stimulates the exchange of electrons between the surface and the sample.
The flow of electrons is measured as a current known as the Faradaic current. This current is plotted against the voltage to produce a voltammogram. Electrochemists analyze the resulting current-potential plot to determine the concentrations of specific analytes, typically by choosing a working electrode that selectively interacts with the target analyte. The currents observed in a voltammetric experiment are valuable because they are directly proportional to the concentration of the target species.
Electrochemical Methods in Forensic Science
Electrochemistry ranks higher among analytical methods due to its applicability and its scope in science. From a range of applications such as in solar cells to biosensors, the industrial uses are immense.
In forensics, the more prominent applications include poison or drug detection in samples, detection of fingerprints, and much more.
1. Poisons
Today, forensic electrochemistry is employed to detect poison concentrations right at the crime scene using printed carbon electrodes.
In a particular poisoning case in Scotland, atropine was tested electrochemically using a voltammetric method. The reaction between atropine and the working electrode, a screen-printed electrode (SPE) was found at a potential of +0.82 V in a pH 10 model buffer solution.
This allows us to move the lab into the field for on-site analysis, which will cut down on the amount of time it takes to receive result feedback and lower the possibility of sample contamination across locations.
Using inexpensive, disposable, and repeatable electrodes that don’t need to be pretreated in the lab before analysis, the printed electrode setup has the advantage of miniaturizing the electrochemical approach. SPEs are used in several forensic electrochemistry applications. These benefits replace costly and time-consuming laboratory methods like gas chromatography with mass spectrometry and high-performance liquid chromatography.
2. Fingerprinting

Electrochemistry can also be applied in forensic fingerprinting through innovative techniques. One study demonstrated that metal surfaces exhibit varying corrosion rates when they come into contact with fingerprints. Using electrolysis, the researchers “created” a fingerprint on a metal surface by immersing it in a hydrochloric acid solution. The fingerprint acts as a corrosion barrier, causing the metal’s exposed areas to corrode faster than the areas covered by the fingerprint. When a reductive voltage is applied, electrochemical reactions corrode the surface, etching the fingerprint into the metal.
This method was further tested on a bullet fired from a gun, addressing the issue that high temperatures and pressures can destroy fingerprints, making them undetectable by conventional methods. The technique showed success, with the etched fingerprint becoming visible after just 5 minutes of electrolysis. This approach could be easily applied in the field using dilute hydrochloric acid and a handheld potentiostat, potentially supporting criminal investigations when traditional fingerprint methods fall short.
Conclusion
Forensic electrochemistry offers a wide array of applications, ranging from poison and drug detection to fingerprint mapping, addressing the current need to detect chemical and physical components across diverse forensic scenarios. The key advantages of electrochemistry include the potential for handheld point-of-care (PoC) devices, low detection limits, a broad range of detectable species, and ease of use. These benefits make it a promising field for developing more accurate and reliable technologies for forensic analysis. With its unique combination of portability, selectivity, sensitivity, and cost-effectiveness, forensic electrochemistry is poised for significant growth, likely expanding its range of applications and establishing itself as a robust analytical platform.
References
An Introduction to Forensic Electrochemistry
Forensic electrochemistry: developing electrochemical sensors for the detection of illicit compounds

