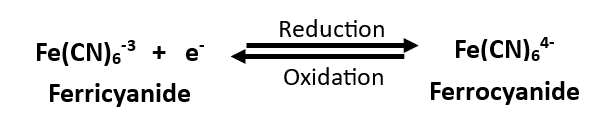
This application note provides detailed instructions for using the MedPstat device in Ferro/Ferri cyanide redox couple investigations, employing MTX Labs Screen-printed carbon electrodes. This Ferro/Ferri cyanide redox system serves as an excellent model for understanding cyclic voltammetry and electrochemical processes.
What you will learn in this Application Note?
- What is SPE & why is it used for?
- How to connect an SPE with a Potentiostat?
- How to add an appropriate solution to SPE?
- How to perform a cyclic voltammetry with SPEs?
- How to interpret a cyclic voltammogram from SPCEs?
- How to overlay cyclic voltammograms for different scan rates in MedEplot software?
Introduction:
Cyclic voltammetry (CV) is a fascinating technique used by electrochemical researchers. It involves applying a voltage to an electrochemical cell and observing the resulting electric current as the voltage is systematically varied back and forth. This method provides crucial insights into oxidation and reduction potentials, the number of electrons involved in redox reactions, and the kinetics of these reactions. Now, let’s talk about our MTX Labs screen-printed electrodes. These electrodes offer significant advantages, particularly for researchers. They are remarkably cost-effective, making them accessible even to those on a limited budget. Moreover, they are incredibly space-efficient, fitting snugly into small laboratory settings. Additionally, screen-printed electrodes require minimal sample material, making them ideal for researchers just beginning their explorations or those with limited sample availability. By combining the power of cyclic voltammetry with the affordability and ease of use of screen-printed electrodes, researchers can embark on exciting electrochemical investigations.
Experimental:
Reagents:
Prepare a 5 mL stock solution in deionized water containing 5 mM K3Fe(CN)6 and 5 mM K4Fe(CN)6 in 1 M KCl.
Apparatus:
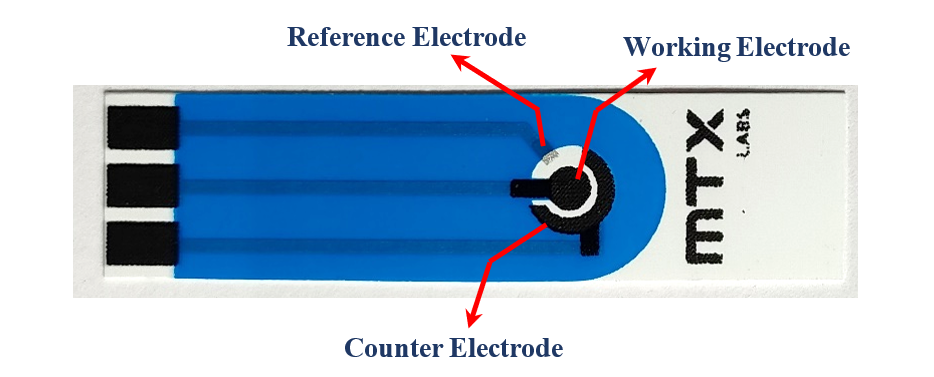
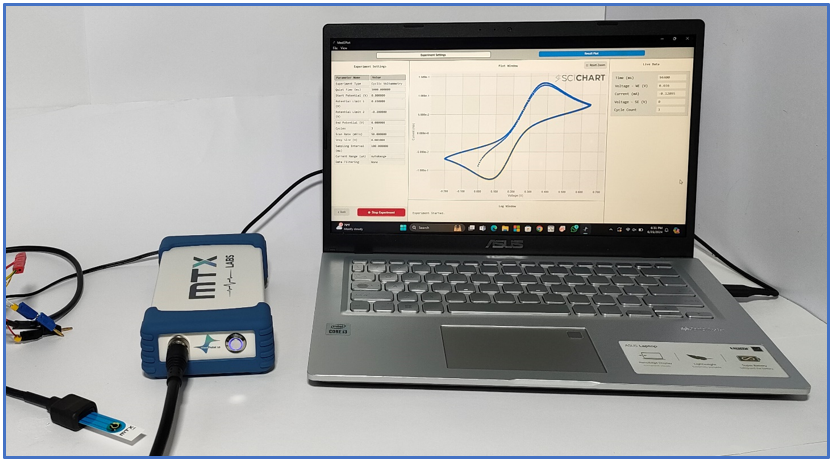
In our experiment, we used a handy device called the MedPstat, which is a portable and compact potentiostat that operates on the “MedEplot” software compatible with Windows. For the electrochemical reaction, we employed Screen Printed Carbon Electrodes sourced from MTX Labs. These electrodes comprise three essential components: an Ag/AgCl ink serving as the reference electrode, carbon utilized as the counter, and a working electrode. Before experimenting, it’s recommended to thoroughly rinse the electrode surface with deionized water and delicately wipe them using a tissue. This ensures proper preparation of the electrodes, which is crucial for achieving accurate and reliable results in our experiment. The connection scheme between the potentiostat and the SPE connecting wire is identified by Red for the working electrode (WE), Blue for the reference electrode (RE), Black for the counter electrode (CE), and Green for Ground (GE).
Procedure:
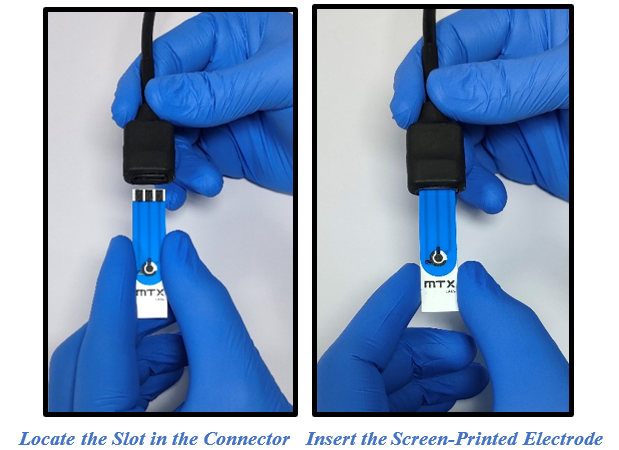
- Identify the appropriate slot on the connector for the screen-printed electrode insertion. Make sure the connector is correctly oriented to accommodate the electrode. Carefully align the screen-printed electrode with the designated slot, and gently push it in until it is securely positioned.
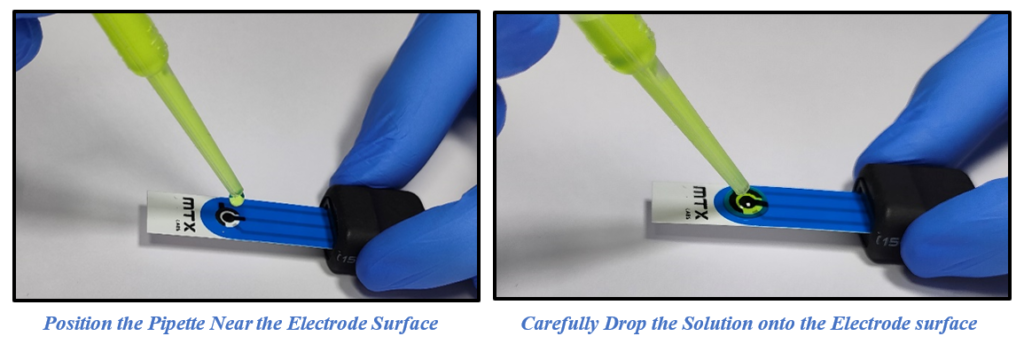
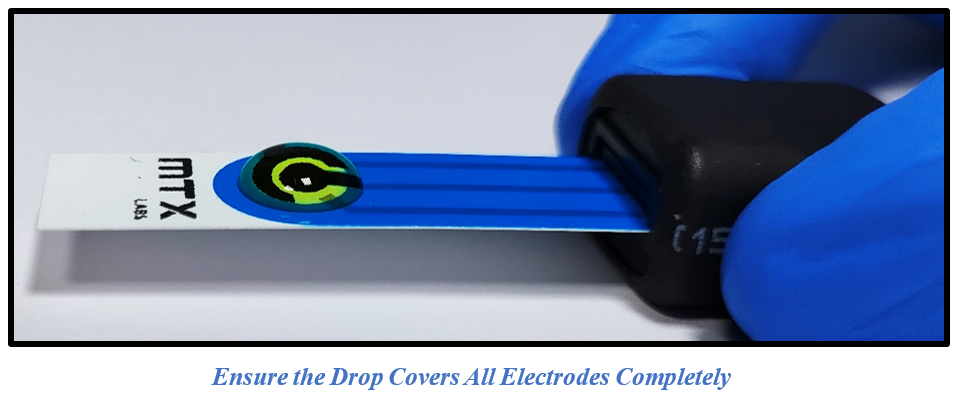
2. Introduce ~100µl of a 5mM Ferro/Ferri cyanide solution into the MTX Labs Screen Printed Carbon Electrodes (SPCE), and gently dispense the solution without causing splashes or bubbles. Ensuring there is sufficient volume for the electrolyte to be covered in the electrode surface. Connect the SPE electrode wire with MedPStat following the previously provided instructions.

- Press the Cyclic Voltammetry (CV) technique. Upon choosing cyclic voltammetry, the window divides into two sections. On one side, you’ve got the General Parameters box next to the experiment techniques, and on the other, you’ll find the fundamentals of cyclic voltammetry.
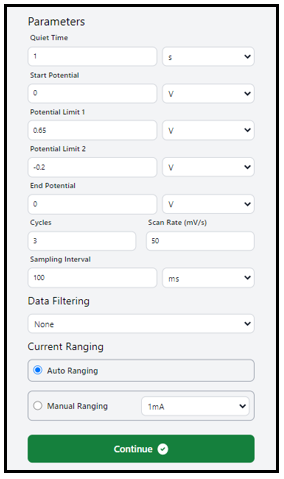
4. Simply click on the dialogue box to modify the values as needed. Follow the suggested settings or feel free to customize them, as demonstrated in the figure below. Press “Continue” to kick off the experiment. Once you do, a new window will pop up.

5. Initiate the experiment by clicking on “Start Experiment.”
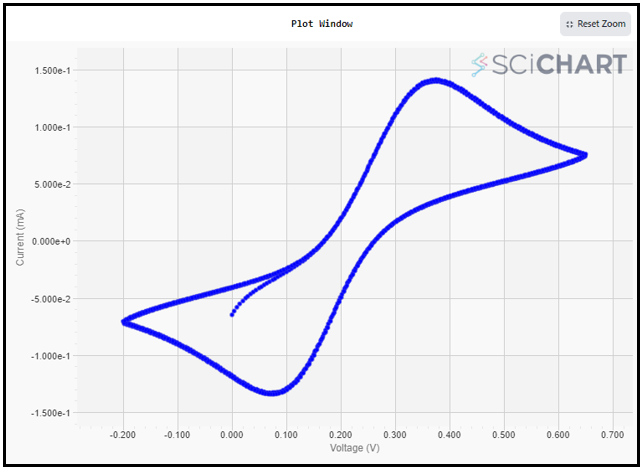
6. The voltammogram will appear on the screen as it is produced. and it’ll adjust itself automatically when done. Here are the cyclic voltammetry results for 5mM Ferro/Ferri cyanide in 1M KCl for 2 cycles.
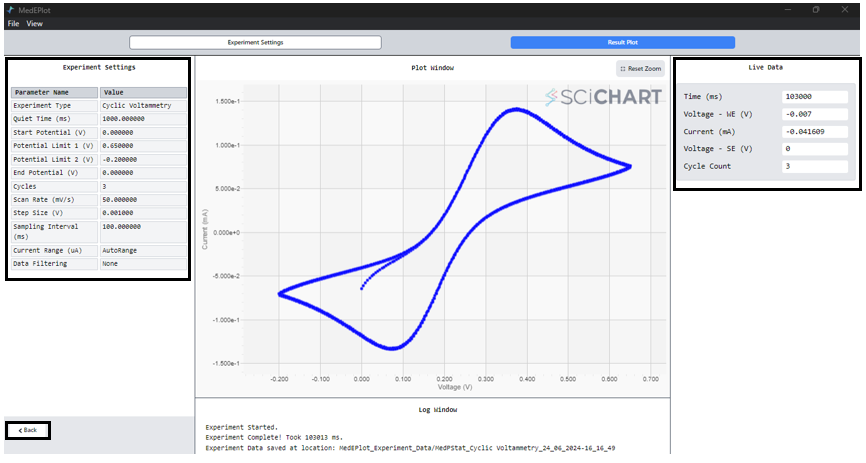
7. In the Cyclic Voltammogram window, access experimental settings on the LEFT, observe real-time data on the RIGHT, and find the completion status at the bottom. To return to the main window or initiate another experiment, simply press the ‘BACK’ button.

8. After identifying both the anodic and cathodic peaks, simply click on the arrow symbol adjacent to the peak to access the numerical values associated with these peak parameters.
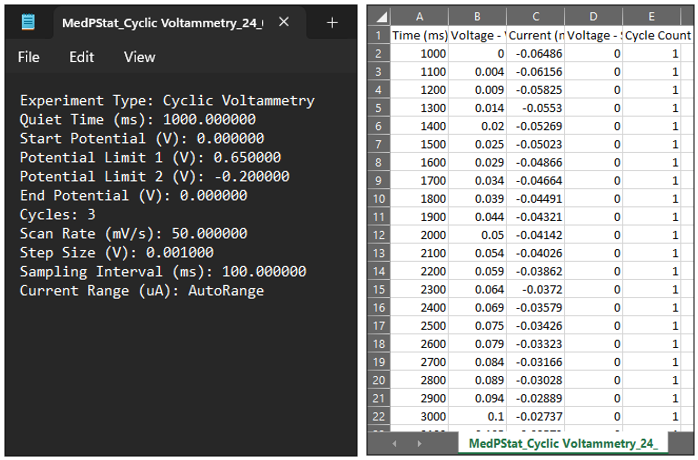
9. Check saved Data on PC: Once the experiment is completed, navigate to the MedEPlot Experiment Data folder in the Documents folder. Within this folder, you will find two sets of files: one comprising the experimental data file (.txt), and the other consisting of the Excel sheet data file (.csv).
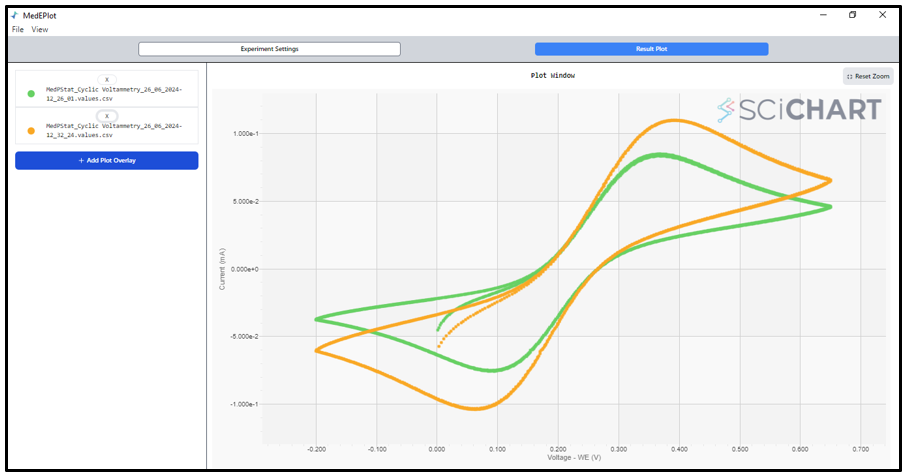
10. To overlay plots, navigate to ‘File’ > ‘New Data’ > ‘Open Data from CSV File’. Once the data is opened, select ‘New Overlay Plot’ to add additional plots for comparison. The graph provided serves as a reference. We conducted cyclic voltammetry (CV) at various scan rates: 20, and 40 mV/s.
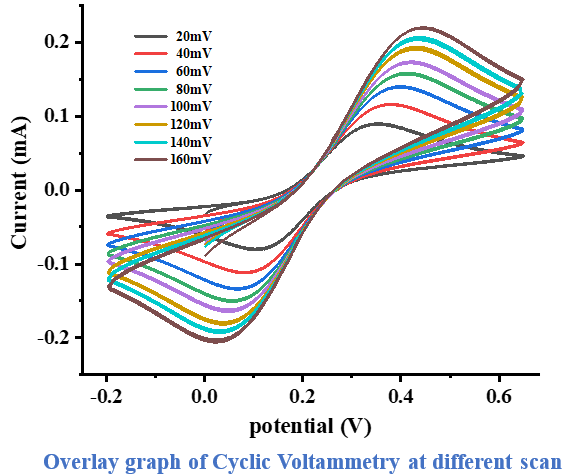
11. We performed the measurements at intervals ranging from 20 mV to 160 mV in increments of 20 mV. This allows us to observe the diffusion-controlled behavior of the redox reactions occurring in the ferro/ferri cyanide solution under different conditions. The resulting data was plotted using Origin 2018 software. By analyzing these graphs, we gain valuable insights into how the scan rate influences the peak locations of the cyclic voltammogram.
Conclusion:
In this application note, we discussed key aspects of using Screen-Printed Electrodes (SPEs) in cyclic voltammetry. Our aim is to provide a comprehensive and accessible guide for researchers exploring electrochemical studies using SPEs. These compact, disposable electrodes are well-suited for a wide range of electrochemical analyses, offering convenience and portability. By the end of this note, readers will have a solid understanding of effectively integrating SPEs into their electrochemical research, enhancing both the accuracy and efficiency of their experimental procedures.
Thanks for Choosing MTX Labs as your research companion.
MTX Labs Team

