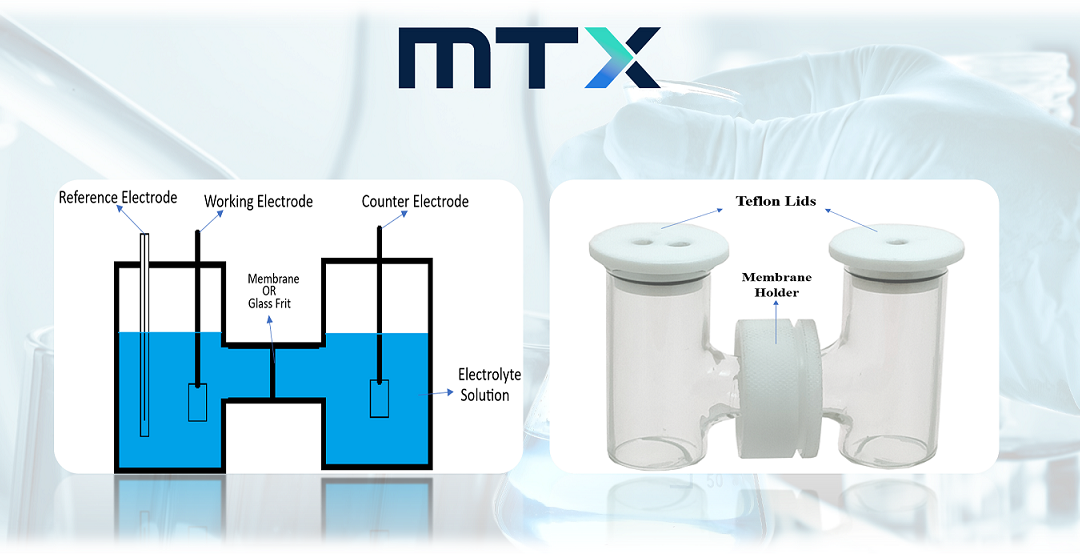
What is an H-Cell?
H-cell is a dual-compartment cell that is mainly designed for electrochemically characterizing a membrane or any kind of separator in a liquid medium. The typical characterizations include measurement of selectivity, cross-over, resistance, and conductivity. H-cells are used alongside electrochemistry in various situations where it’s important to keep the working electrode separate from the counter electrode.
Applications of H-Cell
Here are some examples of H-Cell Applications:
- Potentiometric Studies:
In these studies, H-cells are used to isolate the two reactions so that we can measure the potential difference accurately without any unwanted influences.
- Synthesizing Compounds:
When creating new compounds, H-cells help keep the products, which form at the working electrode, separate from any by-products that might form at the counter electrode.
- Microbial Electrolysis:
In microbial electrolysis, H-cells are utilized to ensure that what’s happening at the working electrode doesn’t mix with what’s occurring at the counter electrode. This separation is essential.
In general, both compound synthesis and microbial electrolysis use a technique called bulk electrolysis, which helps to create new substances by changing functional groups or metal centers. The separation between the working and counter electrodes in these setups offers two key advantages:
- Minimizing Interference: It reduces the chances of unwanted substances produced at the counter electrode affecting the desired reactions at the working electrode.
- Easier Product Collection: Especially when dealing with gases, separating the two electrodes makes it much simpler to collect and handle the final products.
Types of H-cells
H-Cells are mainly categorized into two types
1. H Cell (Separator with Membrane Holder)
The cell comes without any prefixed membrane in it. User can easily open the membrane holder & fix their membrane. A typical product example can be studied & purchased from following link.
2. H Cell with Glass Frit Separator
In this cell both the compartments are separated by a sintered glass frit which stops bulk mixing of the liquids in both the compartments. A typical product example can be studied & purchased from following link.

Difference between membrane and glass frit
Membranes are selectively permeable barriers that allow a particular chemical species like ions, to pass through. The electrolyte of Li-ion batteries, for example, can be thought of as a membrane since it prevents electron passage between the anode and the cathode while allowing Li ions to pass through.
Example: zirfon® membrane &EPDM ZrO2 selectively transport anions OH– ions
EPDM CaCO3 membrane transport CO32- (carbonate anions)
| Feature | Membrane | Glass Frit |
| Material | Polymer or ceramic | Porous glass |
| Size | 0.2-10 µm | 10-100 µm |
| Porosity | 70-90% | 50-70% |
| Ion selectivity | High | Low |
| Resistance | Low | High |
| Cost | High | Low |
| Applications | Biological experiments, fuel cells, batteries | General electrochemical experiments |
As you can see, the main difference between a membrane and a glass frit is their ion selectivity. Membranes are more selective than glass frits, which means that they allow only certain ions to pass through them. This makes them ideal for biological experiments and other applications where it is important to control the ion composition of the solution. However, membranes are also expensive and have a higher resistance than glass frits.
How do I setup my H-cell experiment?
H-cell is used to perform electrochemical measurements through various techniques such as cyclic voltammetry, chronoamperometry, impedance spectroscopy etc.
In the utilization of an H-cell, it is a fundamental requirement to have three essential components: a counter electrode, a working electrode, and a reference electrode, all immersed in an electrolyte solution.
Preparation of electrolyte solution
- Choose the desired electrolyte salt, like sodium chloride (table salt) or potassium sulphate.
- Measure a specific amount of the chosen salt (usually in grams) and add it to distilled water.
- Stir the mixture until the salt fully dissolves in the water.
- Your electrolyte solution is ready for use in the electrochemical cell.
in a CV experiment, as electrons transfer, ions in the solution move to maintain electrical balance. To reduce solution resistance, a salt (supporting electrolyte) is added to the solvent, forming the “electrolyte solution.” Here’s what makes a good solvent and supporting electrolyte:
Solvent:
· Fully dissolves the analyte and supporting electrolyte.
· Stable against oxidation and reduction within the experiment’s range.
· Doesn’t cause harmful reactions with analyte or electrolyte.
· Can be purified if needed.
Supporting Electrolyte:
· Highly soluble in the chosen solvent.
· Chemically and electrochemically inert under experimental conditions.
· Can be purified as necessary
Working electrode:
- The working electrode plays a central role in electrochemical experiments. It is used to control the applied potential and must be made of a non-reactive material within the desired potential range.
- To ensure accurate results, the working electrode must have a clean and precisely defined surface. Typically, this involves mechanical polishing and sonication in ultrapure water. Multiple CV scans across a wide potential range may be needed to eliminate any residual adsorbed species, a process known as “pre-treating” the electrode.
- For glassy carbon electrodes, which are highly reactive once activated, treating the solvent with activated carbon can help prevent impurities from adsorbing to the electrode surface.
- Electrodes may need to be repolished between measurements, especially if analytes tend to adsorb to the electrode surface. A rinse test can determine if an analyte is strongly adsorbed, and separate polishing pads are advisable before and after experiments to avoid contamination.
- Different electrode materials can lead to varying electrochemical responses due to differences in electron transfer kinetics, adsorption tendencies, or reactivity with substrates. Changing the electrode material can help diagnose and address these issues.
- A reference electrode serves as a stable reference point for measuring the potential of other electrodes in electrochemical cells. Common ones in aqueous solutions include the saturated calomel electrode (SCE), standard hydrogen electrode (SHE), and AgCl/Ag electrode, separated from the solution by a porous frit. Matching solvent and electrolyte with the experiment minimizes junction potentials.
- In non-aqueous solvents, Ag+/Ag-based reference electrodes are used, with the potential varying between experiments due to factors like Ag+ concentration or solvent changes. To address this, it’s recommended to reference data to an internal compound with a known E0′, like ferrocene at 0 V vs. Fc+/Fc.
- To prevent Ag+ leakage, one option is to use a silver wire pseudo reference electrode without Ag+ salt, separated from the analyte by a frit, which relies on the natural oxide layer on the silver wire. Regardless of the reference choice, having an internal standard like ferrocene in all measurements is advisable.
- The counter electrode in an electrochemical setup completes the electrical circuit. It ensures that current can flow as electrons move between the working electrode (WE) and the counter electrode (CE). Typically, platinum or carbon-based materials are used as counter electrodes.
- To prevent any interference with the reaction at the working electrode, the surface area of the counter electrode is larger than that of the working electrode. The CE should also be chosen to be inert to avoid generating unwanted by-products.
References: A Practical Beginner’s Guide to Cyclic Voltammetry Noemie Elgrishi, ́ Kelley J. Rountree, Brian D. McCarthy, Eric S.Rountree, Thomas T. Eisenhart, and Jillian L. Dempsey