This application note provides a comprehensive guide for assessing the performance and stability of the MedPstat Electrochemical Analyzer. The Ferro/Ferri cyanide redox couple was chosen as a model system due to its well-defined and reversible electrochemical behavior, making it an ideal standard for evaluating cyclic voltammetry, chronoamperometry, and Galvanostatic charge/Discharge experiments.
Introduction:
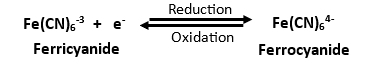
The Importance of Potentiostat Stability. In this study, we used the MedPstat Electrochemical Analyzer to evaluate its stability, reliability, and durability for extended electrochemical experiments. To systematically examine the long-term reliability of the MedPstat, we conducted three key electrochemical tests: cyclic voltammetry (CV) for 10,000 cycles to assess the reproducibility of redox peak potentials and current responses, chronoamperometry (CA) for 24 hours at a fixed potential to evaluate current stability, and Galvanostatic charge-discharge (GCD) for 5000 Cycles under constant current conditions. These experiments provide useful insights into the robustness of the MedPstat, demonstrating its capability for high-precision electrochemical measurements in both research and industrial applications.
Experimental:
Reagents:
Prepare a 50 mL stock solution in deionized water containing 5 mM K3Fe(CN)6 and 5 mM K4Fe(CN)6 in 1 M KCl.
Apparatus:
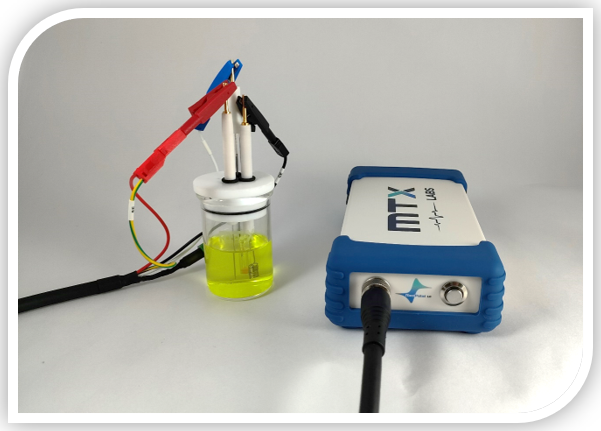
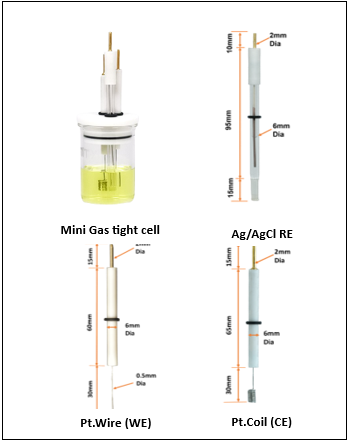
In this experimental arrangement, we employed the MedPstat, a portable and compact potentiostat that operates on the “MedEplot” software compatible with Windows. Notably, the software includes a Device status indicator, where Grey signifies no connection, Blue indicates readiness for connection, and Green denotes a connected device. For the electrochemical reaction, a 3-electrode voltammetry cell (MTX Labs mini gas-tight cell) was utilized, consisting of a Platinum wire electrode (MTX Labs, India) with an outer diameter of 6mm and an inner diameter of 3mm, a Silver/Silver chloride reference electrode (MTX Labs, India) measuring and 6mm in diameter, and a Platinum Coil counter electrode with a length of 30mm. Before experimenting, rinse the electrodes with deionized water and gently wipe them with a tissue. The connection scheme between the potentiostat and the cells is identified by Red for the working electrode (WE), Blue for the reference electrode (RE), Black for the counter electrode (CE), and Red for Sensing (SE).
Result and Discussion:
Introduce 30 mL of a 5 mM Ferro/Ferri cyanide solution into the MTXC02 Mini gas-tight cell, ensuring there is enough volume for the electrodes to be immersed. Submerge the electrodes in the cell, and if desired, insert an optional purging tube. Connect the electrodes according to the previously provided instructions.
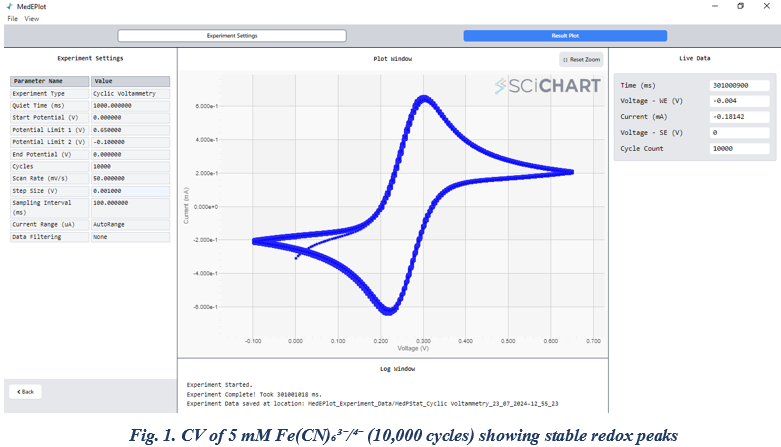
We performed cyclic voltammetry (CV) within a potential range starting from 0 V, with potential limit 1 set at -0.65 V, potential limit 2 at -0.1 V, and the end potential at 0 V, at a scan rate of 50 mV/s. The experiment was conducted for 10,000 cycles, as shown in Figure 1. On the left side of the figure, the live data display shows the total time taken to complete the experiment, which was approximately 301,000 seconds (around 3.5 days). Throughout the experiment, the instrument operated without any interruptions, system hangs, or malfunctions, demonstrating its stability and reliability for extended electrochemical measurements.
Chronoamperometry (CA) :
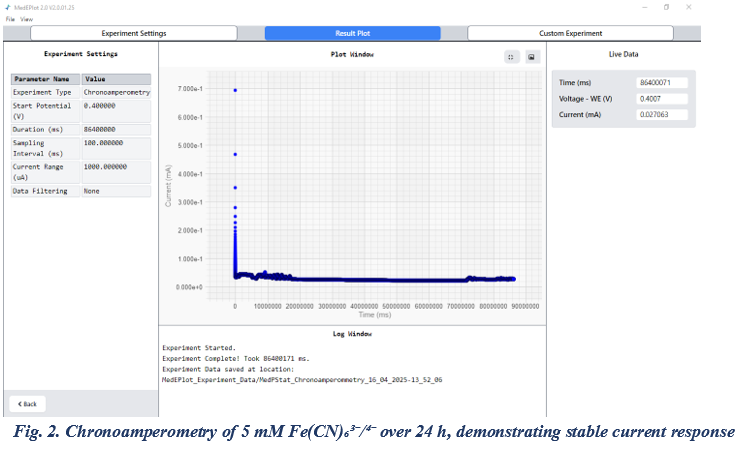
In our second experiment, chronoamperometry (CA) was performed at a constant applied potential of 1 V for 24 hours to assess the long-term stability of the MedPstat electrochemical workstation. The experiment was conducted in the same 5 mM ferro/ferri cyanide solution. As shown in Figure 2, the current response remained stable throughout the test, with no significant drift or fluctuations observed. This indicates the workstation’s ability to maintain a steady electrochemical response over extended periods. The results validate the reliability and consistency of the MedPstat under prolonged operational conditions, confirming its suitability for high-precision and long-duration electrochemical studies
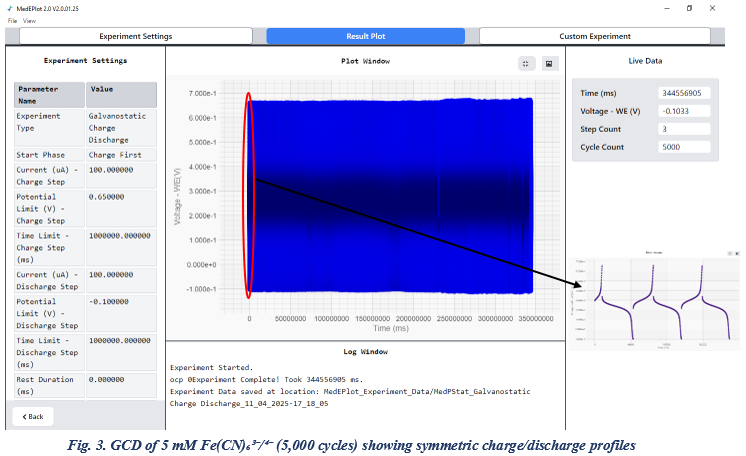
Galvanostatic Charge–Discharge (GCD) :
To further evaluate the long-term stability and performance of the MedPstat electrochemical workstation, 5000 cycles of Galvanostatic Charge–Discharge (GCD) measurements were performed using the ferro/ferricyanide redox couple. This extended cycling test serves as a robust indicator of the system’s durability and its ability to deliver consistent electrochemical responses under repeated operation. While a representative GCD profile is shown, to enhance visualization, a magnified inset is included within the main graph. Additionally, the experimental parameters can be observed on the left side of the image for reference.
Result:
The long-term electrochemical tests conducted using the MedPstat demonstrated its excellent stability and reliability. The system successfully performed cyclic voltammetry (CV) for approximately 3.5 days, chronoamperometry (CA) for 1 day (with the capability to extend experiments up to 7 days), and Galvanostatic Charge–Discharge (GCD) for 3.9 days. In the GCD tests, we completed 5,000 cycles, though the system is capable of handling up to 10,000 cycles without any issues. Throughout all experiments, the instrument operated without any software errors, system hangs, or interruptions. These results confirm that the MedPstat is highly suitable for extended electrochemical studies, making it a dependable tool for both routine and advanced research applications.
Thanks for choosing MedPstat as your research companion.
MTX Labs Team