- Make sure the electrode is free from contamination and it is clean.
- To achieve equilibrium, immerse the electrode in a 3M (KCl/NaOH) solution.
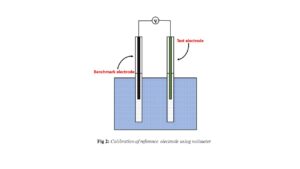
- Choose the standard reference electrode with known potential.
- As illustrated in Fig 1, place both electrodes in a cell containing saturated 3M (KCl/NaOH) and connected to a voltmeter. It is useful for determining the potential of the reference electrode.
- If the voltage value is zero, the reference electrode is functioning properly.
- If the voltage value is non-zero or near to zero, the difference is very small and it may be acceptable.
- If the voltage value fluctuates more than usual (unstable), take it into consideration, and then verify the concentration of the filling solution to restore the electrode’s condition
.